The New Cancer Therapy Called CAR-T
By Eva Briggs, M.D.
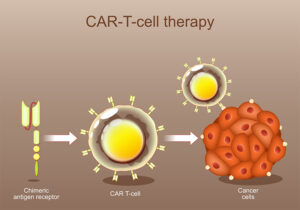
At a recent medical conference, I listened to a talk about a relatively new cancer therapy called CAR-T. This stands for chimeric antigen receptor T-cell therapy.
I found it very interesting, since my brother died young from leukemia and perhaps if this treatment had been available 20 years ago, his life might have been prolonged. CAR-T was first approved in 2017.
Cancer cells are often sneaky, spreading throughout the body. If they just stayed put and didn’t metastasize, it might be possible to treat and cure the original cancer with surgery or radiation. But finding cancer cells dispersed throughout the body is a challenge. CAR-T uses a patient’s own specially modified immune T-lymphocyte cells (T cells for short) to ferret out specific cancer cells and destroy them.
Cells have proteins on their surface called antigens. T-cells have proteins on their surface called receptors. When a receptor and antigen match — like fitting the correct key into a lock — the T cell can bind to the matching cancer cell. Once bound, it releases its immune weaponry to destroy the cell.
CAR-T cells are engineered from the body’s own T-cells to bind to antigens found on cancer cells. First a patient’s T cells are removed from the blood in a process called leukapheresis. Blood is drawn off by an IV catheter and passed through a machine that separates out the white blood cells. The remaining blood is then returned by IV. This process takes several hours as an outpatient. Sometimes a second session is needed to collect a sufficient number of cells. The process may cause a patient’s calcium to drop causing numbness and tingling. Supplemental calcium by mouth or IV treats low calcium. The harvested white blood cells are frozen and shipped to a special laboratory.
In the lab, T-cells are engineered to have receptors capable of binding the specific antigen found on the surface a patient’s cancer. These new cells are the CAR-T cells. The lab grows and multiplies these cells until there is a sufficient number for the treatment. This process may take several weeks.
Once the CAR-T cells are ready, the patient may first receive a short course of chemotherapy. This lowers the body’s immune cells so that they will be less likely to attack the infused CAR-T cells. But the goal is to leave a sufficient number of cancer cells for the CAR-T cells to find and destroy. The CAR-T infusion takes about an hour, during which the patient is monitored for a reaction.
Once the CAR-T cells begin binding to cancer cells, they multiply and attack even more cancer cells. This process occurs over weeks, so patients are monitored closely for several months to detect any adverse reactions. Some of these side effects are potentially serious.
When the CAR-T cells multiply they release inflammatory chemicals called cytokines. Large quantities can lead to cytokine release syndrome (CRS). CRS is characterized by fever, chills, trouble breathing, nausea, vomiting, diarrhea, headaches, lightheadedness, racing heart and exhaustion. Fortunately it’s treatable with a medicine called toclizumab and steroids.
Another serious side effect is immune effector cell-associated neurotoxicity syndrome (ICANS). Symptoms are headache, confusion, decreased level of awareness, seizures, shaking, trouble speaking, and impaired balance. Due to the risk of ICANS, patients cannot drive, operate heavy machinery or do other hazardous activities for at least eight weeks following treatment.
Additional possible side effects are allergic reactions during the infusion, altered levels of blood chemicals, decreased blood cell counts, and the risk of developing a second cancer.
CAR-T therapy can knock out some cancers that have failed other treatments, in some case leading to long term remission. But that same power means it also can cause serious side effects. Another problem is the cost. The total cost after manufacturing the CAR-T cells, hospital admissions, lab tests, medicines and doctor visits can exceed $500,000! And it is often not fully covered by insurance.
Still, CAR-T is a promising treatment for some tough cancers, and future developments and refinements are likely for this relatively new therapy.

